Plant tissue culture is a powerful tool for regenerating plants from small tissue samples under controlled conditions. One of the key factors that significantly influences the success of plant tissue culture is the composition of the growth medium, especially the balance of plant hormones or plant growth regulators (PGRs). Understanding how different hormones interact with the plant tissue is critical for optimizing the growth medium and achieving efficient regeneration.
In this blog, we’ll explore the role of plant hormones in tissue culture, how they influence various stages of plant development, and how to fine-tune their concentrations to achieve the best results.
The Role of Plant Growth Regulators in Tissue Culture
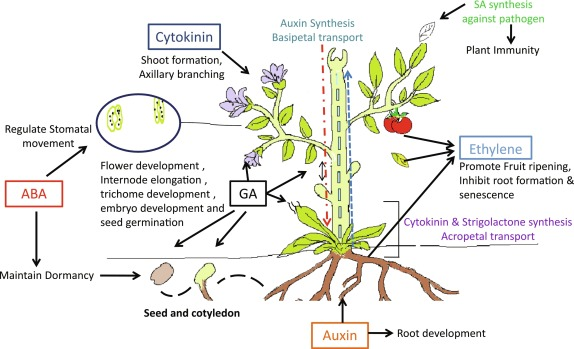
Plant growth regulators (PGRs) are chemicals that influence plant growth and development. In tissue culture, two main categories of hormones are most commonly used: auxins and cytokinins. While these two classes of PGRs are central to plant regeneration, other regulators like gibberellins, abscisic acid (ABA), and ethylene can also play roles depending on the plant species and tissue type.
The balance between auxins and cytokinins is particularly important for determining the developmental pathway that cultured tissues will follow. Manipulating this balance allows researchers to induce the formation of roots, shoots, or callus (an undifferentiated mass of cells).
Key Hormones in Plant Tissue Culture
- Auxins
Auxins, such as indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and naphthaleneacetic acid (NAA), are primarily involved in promoting cell elongation, root formation, and callus induction. In tissue culture, auxins are often added to stimulate the formation of callus and roots from explants.- Callus Induction: Auxins are critical for callus formation from plant tissues. A higher concentration of auxin relative to cytokinin generally promotes the induction of callus, which can be further manipulated to regenerate shoots or roots.
- Root Formation: When the auxin concentration is high relative to cytokinins, root formation is typically favored. For example, IBA and NAA are commonly used to induce rooting in cultured plantlets during micropropagation.
Catalog Number Name IBAPW Indole-3-Butyric Acid (IBA) Powder IBA100 Indole-3-Butyric Acid (IBA) Solution NAAPW Naphthaleneacetic (NAA) Powder NAA100 Naphthaleneacetic Acid (NAA) Solution IAAPW Indole-3-Acetic Acid (IAA) Powder IAA100 Indole-3-Acetic Acid (IAA) Solution - Cytokinins
Cytokinins, such as 6-benzylaminopurine (BAP) and kinetin, are responsible for promoting cell division and shoot formation. Cytokinins counterbalance the effects of auxins and are used to stimulate the development of shoots from callus or explants.- Shoot Induction: When the ratio of cytokinins to auxins is high, shoot formation is favored. This is particularly important in the regeneration of whole plants from undifferentiated tissue.
- Cell Division and Organogenesis: Cytokinins promote rapid cell division, which is essential during the organogenesis phase where specific organs (shoots, roots) develop from callus tissue.
- Gibberellins (GA)
Gibberellins are involved in promoting stem elongation and seed germination. While less commonly used in tissue culture, gibberellins can be beneficial in specific cases, such as enhancing the elongation of regenerated shoots.- Shoot Elongation: In some cases, gibberellins are added to promote the elongation of shoots, especially when cytokinin-induced shoots are stunted or have inhibited growth.
- Abscisic Acid (ABA)
Abscisic acid is typically associated with stress responses and the induction of dormancy in seeds. In tissue culture, ABA is often used to induce somatic embryos to become dormant, thus preserving them for future growth. - Ethylene
Ethylene is a gaseous hormone that can have both positive and negative effects in tissue culture. While it can promote callus formation and stress responses, excessive ethylene production can inhibit shoot development, making it a hormone that needs to be carefully regulated.
Fine-Tuning the Hormonal Balance for Optimal Growth
The success of plant tissue culture depends on achieving the correct balance of auxins and cytokinins, as well as adjusting the concentrations of other hormones based on the specific needs of the plant species being cultured. Here are some general guidelines for optimizing hormone levels during different phases of plant tissue culture:
- Callus Induction
- Auxin: High | Cytokinin: Low
- A high concentration of auxins, such as NAA or 2,4-D, is essential for inducing callus formation. The auxin-to-cytokinin ratio should be skewed toward auxins to encourage cell proliferation and the development of an undifferentiated cell mass.
- For example, a typical medium for callus induction might include 2 mg/L of NAA and 0.5 mg/L of BAP.
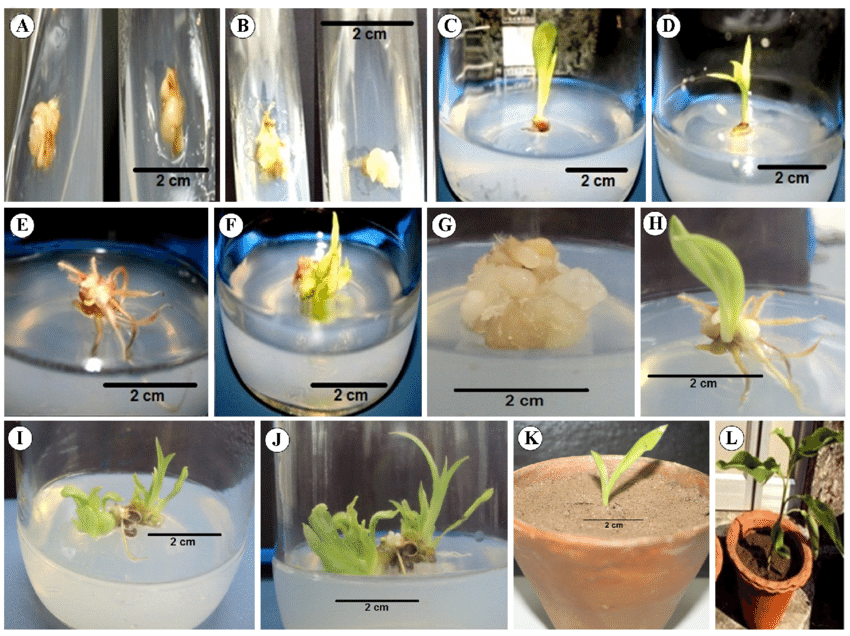
- Shoot Regeneration
- Auxin: Low | Cytokinin: High
- Once callus formation has been achieved, the medium is typically adjusted to increase cytokinin levels while reducing auxin levels. This promotes the differentiation of shoots from the callus.
- A shoot induction medium might contain 0.5 mg/L of IAA and 3 mg/L of BAP.
- Root Formation
- Auxin: High | Cytokinin: Very Low or Absent
- During the rooting phase, auxins are again elevated to encourage root development. Cytokinins are usually minimized or eliminated to avoid shoot formation.
- Root induction media commonly include higher concentrations of auxins like IBA (1-2 mg/L).
Plant Preservative Mixture (PPM): Controlling Contamination in Plant Tissue Culture
One of the significant challenges in plant tissue culture is controlling contamination from microorganisms such as bacteria and fungi. These contaminants can quickly overtake the culture, depriving plant tissues of nutrients and hampering their growth. To address this, a product called Plant Preservative Mixture (PPM) is often incorporated into the growth medium.
PPM is a broad-spectrum biocide and preservative that is used in plant tissue culture to prevent microbial contamination without harming plant cells. Unlike traditional antibiotics, which target specific types of microorganisms, PPM is effective against a wide range of bacteria, fungi, and molds. It can be added to the growth medium at different stages of tissue culture, including:
- During medium preparation: PPM is added to the sterilized medium before the explants are introduced. It helps maintain a sterile environment throughout the culture period.
- In established cultures: PPM can be added directly to the culture containers to prevent contamination from airborne spores or inadvertent introduction of microbes during handling.
PPM’s main advantage is that it is non-toxic to plant cells at recommended concentrations, usually around 0.05-0.2%, depending on the plant species. It ensures that plant tissues have an uncontaminated environment for growth and regeneration, reducing the need for repeated sterilization and minimizing the risk of losing valuable cultures to microbial infections.
The Importance of Medium Composition: Murashige and Skoog (MS) Medium
The Murashige and Skoog (MS) medium is one of the most widely used basal media in plant tissue culture. It provides essential macronutrients, micronutrients, vitamins, and sugars required for plant growth. However, the addition of plant growth regulators is essential for customizing the MS medium to suit specific needs.
In practice, researchers adjust both the macronutrient concentration (e.g., nitrogen) and the hormonal composition depending on the plant species, the tissue type (leaf, root, stem), and the desired outcome (callus formation, shoot regeneration, root formation). For instance, using a half-strength MS medium with adjusted hormone levels is often optimal for sensitive species or tissue types prone to stress.
pH Adjustments and Hormonal Effectiveness
The effectiveness of hormones in the growth medium is also influenced by the pH of the medium. The ideal pH for most plant tissue culture media is between 5.6 and 5.8. If the pH is too acidic or too basic, it can alter the availability and activity of plant hormones, resulting in reduced efficiency of tissue regeneration. For example, auxins are more effective at slightly acidic pH levels, while cytokinins maintain stability over a broader pH range.
Optimizing the hormonal balance in plant tissue culture requires a nuanced understanding of the specific roles that auxins, cytokinins, and other growth regulators play. By adjusting the concentrations and ratios of these hormones in the growth medium, researchers can control the developmental pathways of cultured plant tissues, leading to the efficient regeneration of roots, shoots, or callus. Fine-tuning these hormone levels in combination with the appropriate growth medium composition and pH adjustments ensures the successful propagation of plant tissues in vitro.
Additionally, incorporating Plant Preservative Mixture (PPM) can significantly improve the success rate of cultures by mitigating contamination risks, offering a more reliable process. The next step in advancing tissue culture techniques will likely focus on integrating more advanced hormones, fine-tuning these protocols for different species, and exploring the genetic factors that influence tissue response to hormonal signals.